Ménière’s disease is an inner ear disorder classically consisting of vertigo spells preceded by fluctuating hearing loss and fluctuating ear noise in one ear. Noise in the affected ear can be a loud ringing, roaring, loud horn noise, seashell noise, or another ear noise. In Ménière’s disease, the noise is usually louder in the affected ear just before, during, and immediately following the spells. The vertigo spells are typically a spinning or tilting sensation commonly accompanied by nausea, vomiting, and sometimes diarrhea and/or marked sweating (diaphoresis). The duration of spinning varies from 20 minutes to several hours. Most persons find a position or place to lie down to try to sleep it off, awakening hours later much improved. However, quick movements may trigger imbalance, unsteadiness, or other dizzy symptoms for hours to a day or so after the Ménière’s spell. The hearing usually improves within hours to a few days in the early stages of Meniere’s disease.
In non-classic forms of Ménière’s disease, the pattern of symptoms may be quite different. In late Ménière’s disease, the hearing may be so poor as not to fluctuate and the ear noise may not increase much, if any, during a spell. Whereas in early to mid- Ménière’s disease, the ear has a pressure sensation before a spell of vertigo, that warning may not be present in late Ménière’s disease. In the classic variant, the hearing loss fluctuates in the low frequencies, whereas in another common variant, the fluctuation is more pronounced in the high frequencies. In the Lemoyez variant, the hearing improves as soon as the vertigo starts, whereas in the classic variant, the hearing takes hours to days to improve. In the Tumarkin’s variant, the vertigo is perceived as falling sensation and the person may drop to the floor. In the vestibular Ménière’s variant, the hearing may remain normal and ear noise symptoms seem not change with the episodes. In the so-called vestibular Ménière’s variant, though, about 80% of patients respond better to migraine management than to classic Ménière’s disease management.
Figure 1 Ménière’s Inner Swelling
©Northwestern University
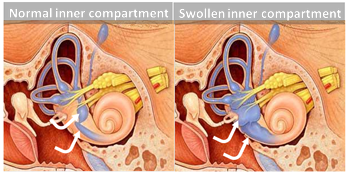
In Ménière’s disease, the acute episodes are caused by rupture of the inner ear’s inner compartment because of high inner ear fluid pressure (Fig 1). This compartment is high in potassium and is surrounded by a high sodium concentration compartment. The pressure problem is thought to increase in the hours to days before the inner ear ruptures. At the time of rupture, mixing of the potassium into the sodium compartment correlates with vertigo onset. With the rupture, the pressure equalizes and the ruptured membrane heals, but re-establishing equilibrium of sodium and potassium may take a while. Imbalance, unsteadiness, and worse balance may last minutes to hours, sometimes days.
The reason for inner compartment swelling is not definitively known. Some believe that dysfunction of the sodium/potassium control systems plays a role. Since newer literature shows that the Herpes simplex viral glycoprotein exists in at least 75% of Ménière’s disease inner ears, our thought is that a protein reaction to viral replication is responsible for the pressure increase.
A variety of symptoms accompany inner compartment swelling: hearing loss, imbalance, ear noise, ear pressure or fullness, sound distortion, and increased sensitivity to loud noises are among the many sensations caused by swelling in the inner ear. Rapid swelling of the inner chamber appears to rupture the thin separating membrane between the two compartments. With a rupture, fluid from the high potassium inner chamber mixes with fluid from the high sodium chloride outer chamber of the inner ear. With sodium intoxication of the sensory cells, hearing declines and a violent vertigo attack starts that may last for minutes to hours. With potassium mixing into the sodium compartment, the balance sensory system becomes dysfunctional, as well.
Some attacks are associated with nausea and vomiting. Variably, the delicate hearing and balance sensory cells suffer both reversible and irreversible intoxication. With each successive attack, injury to the inner ear accumulates. In most cases, the level of function present in a period that has no vertigo spells is the permanent condition but with long term anti-viral treatment, we have seen hearing permanently improve in some.
Treatment Options for Ménière’s Disease
When Ménière’s disease is out of control, the first order of management is to severely restrict sodium chloride intake (table salt). Good evidence exists that during susceptible periods, salt loading correlates with more frequent vertigo spells and that lowering of salt intake correlates with an early decrease in vertigo spells and with less hearing fluctuation. [1], 25 The thought is that doing so lowers inner ear fluid pressure levels. Anything that will cause fluid retention will typically make the symptoms associated with Ménière’s disease worse. Women may notice their symptoms becoming worse around their menstrual cycle. Symptoms are also commonly but not always worse after eating a salty meal. In other words, if you get away with salt indiscretion once, that is not a sign that your inner ears are not sensitive to dietary salt intake. It appears that Ménière’s inner ears are susceptible to salt-loading at least some of the time.[2]
Medical management of Ménière’s disease includes a strict low-salt diet, diuretics (salt removing medicine), anti-nausea medications, anti-anxiety medications, sedatives, and often, antiviral agents. While some also recommend a variety of vitamins and minerals, no good scientific evidence supports their usage.
Article: Office-based Ménière’s Disease Management
The low-salt diet we recommend for initial care is to reduce total table salt intake to about 750 to 800 mg per day. Once Ménière’s disease has been under control for at least 6 months, you may tolerate 1200-1500mg of salt in a 24-hour period. All foods have sodium to some degree, some more than others. Even food prepared without adding table salt contains sodium chloride. Foods with the highest natural salt content are meats, eggs, and milk products, which is why severely limiting protein intake to a bare minimum is a key to Ménière’s disease management. Salt is often added to foods enhance flavor and in some cases serves as a preservative. Reading labels of foods and becoming familiar with sodium content of fresh foods allows careful salt restriction. For example, milk is a body fluid and has a significant amount of sodium chloride, about 120 mg per 8 ounces, about 180 mg in a typical 12 ounce glass, or 240 mg in a 16 ounce milk shake.
Commonly, diet alone is not sufficient to control symptoms. Thus, a diuretic (water pill) is usually prescribed. Sedatives like meclizine (Antivert or Bonine) suppress imbalance and nausea. Since stress, anxiety, and depression can be triggers (not causes) of Ménière’s disease, treatment may involve sedatives like Valium, Ativan, Xanax (benzodiazepines), or anti-depressants such as tricyclics (amitriptyline, nortriptyline), tetracyclics (mirtazapine), SSRI agents (fluoxetine, citalopram, etc.), and others such as clonazepam (Klonopin) Wafers of Klonopin can dissolve and be absorbed through the tongue without having to swallow.
For acute vertigo event management to suppress nausea and vomiting, consider medications like ondansetron, meclizine, sublingual Ativan, sublingual scopolamine or hyoscyamine, sublingual clonazepam, or rectal suppositories of agents like promethazine (Phenergan). For quite severe episodes, intramuscular or intravenous injections of promethazine or other medications can be administered. When episodes require these medications, other more invasive management as described below may be indicated.
A growing body of peer-reviewed, published research suggests that viruses like Herpes simplex (the fever blister virus) and Herpes Zoster (the shingles/chicken pox virus) are the likely cause of Ménière’s disease.[3], [4], [5] We postulate that acute release of virus-related proteins affect inner ear fluid pressures. For those with a history of cold sores, or with evidence of high viral antibodies from a simple blood test, symptoms can often be improved substantially with long-term antiviral medication. The antiviral medication does not kill the virus but suppresses it by preventing the virus’s ability to infect additional cells. A person needs to be on the medication for a few months to notice any significant improvement. Long-term use of antivirals carries a low risk of depressing white blood cell counts or of inducing an allergy to the medication.
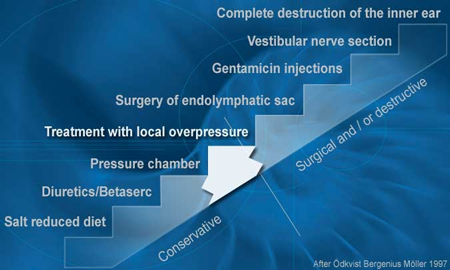
Even without antivirals, over 70% of patients spontaneously improve within 6 months with just diet control and a diuretic. Up to 85% of patients have a spontaneous cessation of vertigo and imbalance problems within 5 years. It appears that adding anti-virals improves the rate of establishing control, improves control rate to 85% [6] much sooner, and decreases the risk of recurrence. Being in a hurry to employ surgery or other invasive management make be overly aggressive management. For the 15-30% who persist with highly troubling vertigo spells and fail to establish early control, invasive treatment options do exist. These other treatments are listed below from the least to the most destructive.
Meniett Device
Endolymphatic Sac Surgery
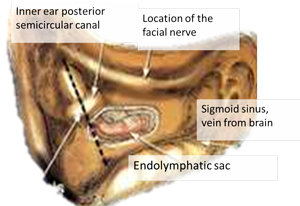
In peer-reviewed published studies, the results of installing a “shunt” or “widely decompressing the sac” or destroying the ELS seem quite similar. We think that whichever the surgery techniques, the internal delicate micro-structure of the ELS is destroyed, removing surface area from which viral activity can release viral proteins, decreasing inner ear fluid pressure. ELS surgery requires an outpatient operation. An incision behind the ear accesses the mastoid bone behind the ear. Drilling the mastoid bone away provides access to the ELS. (See picture)
The surgery takes about an hour to perform. Most people take about a week off work to recover from surgery. The risk of hearing loss or major vertigo or a serious complication is quite low. We think ELS surgery is a good treatment option for patients who have failed maximal medical management and have good hearing. The success rate for vertigo control in patients with early Ménière’s disease is around 70% but long-term success rates are lower. So, 30% of patients may undergo surgery and have no significant improvement in their symptoms. While 30% of operated patients do not have early, complete control of their vertigo spells after ELS surgery, only about 15% of operated ears have enough continuing trouble to require additional surgical procedures.
Chemical Labyrinthine Treatment: Gentamicin or Dexamethasone
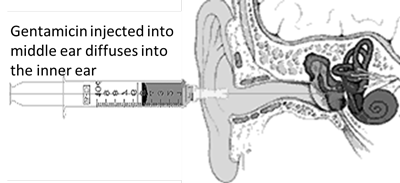
Gentamicin is in a class of antibiotics (aminoglycosides) that have been in use since the 1940s. These drugs are well known to be toxic to the inner ear. In the last two decades, this class of antibiotics has been supplanted by other less toxic drugs, but they are still used to treat life threatening infections. As early as the 1950s, the toxic effects to the inner ear were utilized to treat Ménière’s disease. While the first drug used was streptomycin, it routinely had hearing toxicity when injected into the middle ear. In the 1960s, gentamicin, then used to treat serious infections, was found to be less toxic to hearing than streptomycin. Nevertheless, gentamicin is toxic to the inner ear, comparatively more toxic to the balance system of the inner ear than it is to the hearing system but major loss of hearing may occur. [15], [16], [17], [18] Because of that differential toxicity, it can be used to treat patients with recalcitrant Ménière’s vertigo. The major potential value of the toxicity of gentamicin may be its effect on the cells in the inner ear that secrete fluid and that effect may happen more often than toxicity to hearing and balance sensory cells.
Injecting gentamicin through the eardrum to fill the middle ear allows (see picture) the medication to diffuse into the inner ear. Once the medication is in the inner ear, it takes 2-8 days before it accumulates enough to affect the inner ear cells. Depending on an individual’s sensitivity and how many injections are given, hearing may be permanently affected. Depending on dosage selected and frequency of injections, this treatment is effective in controlling vertigo in 60 to 90% of patients who receive it. [15], [20] Gentamicin treatment is not generally reversible and changes to the inner ear are usually permanent (some spontaneous reversal of toxic effects may occur in some folks receiving a single, lower dose injection [21]). Therefore, a patient should carefully consider his/her options before proceeding with this treatment.
Gentamicin permanently affects the balance system of the treated inner ear. If the balance system in the treated inner ear becomes very weak or non-functional, vertigo control is much more likely. [22], [23] Also, because the treated balance organ becomes weak or non-functional, some permanent, but generally mild, imbalance is expected after the injection. [24], [25] A rare, unusually sensitive patient may experience noticeable vertigo, nausea, vomiting, and dehydration after the injections. Rarely, a brief hospital stay for re-hydration is necessary. After the expected inner ear toxicity develops, most patients experience imbalance with all movement that slowly improves over 2-6 weeks, generally within 4 weeks. Balance and movement exercises started after the injection help restore the balance. Some degree of mild chronic imbalance commonly remains but is virtually always easier to deal with than the vertigo spells.
Vestibular Nerve Section
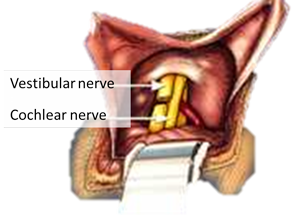
After the acute postoperative vertigo subsides, chronic imbalance lasts for several weeks. The time it takes to regain effective balance is dependent on the individual, age, and amount of activity the patient engages in after surgery. Regaining balance is similar to learning a new language. Younger people have an easier time re-learning balance function than older people, and the more one works on improving balance, the better it becomes. Some have persisting disequilibrium, although less bothersome than the Ménière’s disease type of vertigo. [33], [34],[35] Your doctor will give you a set of exercises to help you regain your balance quicker. Physical therapy for balance is helpful for those who are slow to improve. [36]
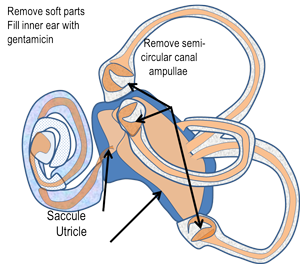
Surgical Labyrinthectomy
Comparison of treatment options in Ménière’s disease
| Treatment | Vertigo Control | Hearing | Time Off Work | Positives | Negatives |
|---|---|---|---|---|---|
| Medical management (low sodium diet, diuretic, antiviral, sedatives, anti-nausea) | 70-80% | Treatment does not cause hearing loss. | No need to be off work | Easy and inexpensive | Takes 4 – 6 wks. to notice any effect |
| Meniett (if medical management fails) | 70% | Requires a tube in eardrum membrane, no change in hearing | No need to be off work | Office procedure, no real risk of hearing loss | Expensive, $3500, not covered by insurance |
| Endolymphatic Sac Surgery (if medical management fails) | 70% | Treatment typically does not cause hearing loss | 1-2 weeks off work on average | Preserves residual hearing | Outpatient surgery, 30% chance of no benefit |
| Middle Ear Gentamicin Perfusion (if medical management fails) | 90%+ | 10-20% chance of worse hearing if 2 or more injections are given. | Off work 0-6 weeks off. | Office procedure | Hearing worse dose-related, imbalance improves with time |
| Vestibular Nerve Section (if medical management fails) | 90% | Worse hearing in 15-30%, some with better hearing | 3 – 6 weeks needed off work | Preserves residual hearing in most patients. | Operation adjacent to brain requiring a short stay in ICU, imbalance improves with time |
| Labyrinthectomy (if medical management fails) | 95% | Complete loss of hearing in operated ear | 2-6 weeks off work. | Very good control of vertigo attacks | Imbalance after procedure improves with time |
References
- Diuretic and diet effect on Menière’s disease evaluated by the 1985 Committee on Hearing and Equilibrium guidelines., , Otolaryngol Head Neck Surg, 1993 Oct, Volume 109, Issue 4, p.680-9, (1993)
- Citekey 25 not found
- Salt-load electrocochleography., , Am J Otol, 1999 May, Volume 20, Issue 3, p.325-30, (1999)
- Herpes simplex virus and Meniere’s disease., , Laryngoscope, 2003 Sep, Volume 113, Issue 9, p.1431-8, (2003)
- The three faces of vestibular ganglionitis., , Ann Otol Rhinol Laryngol, 2002 Feb, Volume 111, Issue 2, p.103-14, (2002)
- Detection of viral antigen in the endolymphatic sac., , Eur Arch Otorhinolaryngol, 1996, Volume 253, Issue 4-5, p.264-7, (1996)
- Ménière’s disease is a viral neuropathy., , ORL J Otorhinolaryngol Relat Spec, 2009, Volume 71, Issue 2, p.78-86, (2009)
- Treatment of Meniere’s disease with the low-pressure pulse generator (Meniett device)., , Expert Rev Med Devices, 2005 Sep, Volume 2, Issue 5, p.533-7, (2005)
- Intermittent pressure therapy of intractable Ménière’s disease using the Meniett device: a preliminary report., , Laryngoscope, 2002 Aug, Volume 112, Issue 8 Pt 1, p.1489-93, (2002)
- Meniett clinical trial: long-term follow-up., , Arch Otolaryngol Head Neck Surg, 2006 Dec, Volume 132, Issue 12, p.1311-6, (2006)
- Effect of transtympanic low-pressure therapy in patients with unilateral Menière’s disease unresponsive to betahistine: a randomised, placebo-controlled, double-blinded, clinical trial., , J Laryngol Otol, 2012 Apr, Volume 126, Issue 4, p.356-62, (2012)
- Effects of middle ear pressure changes on clinical symptoms in patients with Ménière’s disease–a clinical multicentre placebo-controlled study., , Acta Otolaryngol Suppl, 2000, Volume 543, p.99-101, (2000)
- Meniett therapy: rescue treatment in severe drug-resistant Ménière’s disease?, , Acta Otolaryngol, 2005 Dec, Volume 125, Issue 12, p.1283-9, (2005)
- Long-term effects of the Meniett device in Ménière’s disease: the Western Australian experience., , J Laryngol Otol, 2005 May, Volume 119, Issue 5, p.391-5, (2005)
- Longitudinal results with intratympanic dexamethasone in the treatment of Ménière’s disease., , Otol Neurotol, 2008 Jan, Volume 29, Issue 1, p.33-8, (2008)
- Long-term hearing outcome in patients receiving intratympanic gentamicin for Ménière’s disease., , Laryngoscope, 2003 May, Volume 113, Issue 5, p.815-20, (2003)
- Time course of repeated intratympanic gentamicin for Ménière’s disease., , Laryngoscope, 2009 Apr, Volume 119, Issue 4, p.792-8, (2009)
- [The long-term results of treatment of Ménière’s disease with intratympanic injections of gentamicin]., , Otolaryngol Pol, 2012 Jan-Feb, Volume 66, Issue 1, p.20-6, (2012)
- Hearing results following intratympanic gentamicin perfusion for Ménière’s disease., , J Laryngol Otol, 2009 Apr, Volume 123, Issue 4, p.379-82, (2009)
- Intratympanic gentamicin for the treatment of Meniere’s disease and other forms of peripheral vertigo., , Otolaryngol Clin North Am, 2004 Oct, Volume 37, Issue 5, p.1075-90, (2004)
- Intratympanic gentamicin injections for Meniere disease: vestibular hair cell impairment and regeneration., , Neurology, 2002 Nov 12, Volume 59, Issue 9, p.1442-4, (2002)
- Vestibular function and vertigo control after intratympanic gentamicin for Ménière’s disease., , Audiol Neurootol, 2009, Volume 14, Issue 6, p.361-72, (2009)
- Long-term vertigo control in patients after intratympanic gentamicin instillation for Ménière’s disease., , Otol Neurotol, 2007 Dec, Volume 28, Issue 8, p.1140-4, (2007)
- Long-term follow-up of transtympanic gentamicin for Ménière’s syndrome., , Otol Neurotol, 2001 Mar, Volume 22, Issue 2, p.210-4, (2001)
- Transtympanic gentamicin for Meniere’s syndrome., , Laryngoscope, 1998 Oct, Volume 108, Issue 10, p.1446-9, (1998)
- Vestibular neurectomy vs. chemical labyrinthectomy in the treatment of disabling Menière’s disease: a long-term comparative study., , Auris Nasus Larynx, 2009 Aug, Volume 36, Issue 4, p.400-5, (2009)
- Auditory results after vestibular nerve section and intratympanic gentamicin for Ménière’s disease., , Otol Neurotol, 2007 Feb, Volume 28, Issue 2, p.145-51, (2007)
- Vestibular nerve section versus intratympanic gentamicin for Meniere’s disease., , Laryngoscope, 2004 Feb, Volume 114, Issue 2, p.216-22, (2004)
- Vestibular nerve section., , Otolaryngol Clin North Am, 2002 Jun, Volume 35, Issue 3, p.655-73, (2002)
- Vestibular neurectomy with simultaneous endolymphatic subarachnoid shunt., , Eur Arch Otorhinolaryngol, 2002 May, Volume 259, Issue 5, p.243-6, (2002)
- Combined retrosigmoid retrolabyrinthine vestibular nerve section: results of our experience over 10 years., , Otol Neurotol, 2005 May, Volume 26, Issue 3, p.481-3, (2005)
- Vestibular neurotomy by retrosigmoid approach: technique, indications, and results., , Am J Otol, 1991 Mar, Volume 12, Issue 2, p.101-4, (1991)
- Analysis of patients with persistent dizziness after vestibular nerve section., , Ear Nose Throat J, 1998 Apr, Volume 77, Issue 4, p.290-2, 295-8, (1998)
- Transmastoid labyrinthectomy versus translabyrinthine vestibular nerve section: does cutting the vestibular nerve make a difference in outcome?, , Otol Neurotol, 2007 Sep, Volume 28, Issue 6, p.801-8, (2007)
- Clinical assessment of postural stability following vestibular nerve section., , Laryngoscope, 1991 Oct, Volume 101, Issue 10, p.1056-9, (1991)
- The role of vestibular rehabilitation in the treatment of Meniere’s disease., , Otolaryngol Head Neck Surg, 2005 Sep, Volume 133, Issue 3, p.326-8, (2005)
- Endolymphatic sac shunt, labyrinthectomy, and vestibular nerve section in Meniere’s disease., , Otolaryngol Clin North Am, 2010 Oct, Volume 43, Issue 5, p.1091-111, (2010)
- Labyrinthectomy versus vestibular neurectomy: long-term physiologic and clinical outcomes., , Otol Neurotol, 2001 Jul, Volume 22, Issue 4, p.539-48, (2001)